Scientists find link between cancer metabolism and cellular architecture
Researchers at Weill Cornell Medicine and Beth Israel Deaconess Medical Center have discovered a previously unidentified link between a cancer cell's form and shape and its metabolic capabilities.
"This is a very important discovery in that it indicates that the highly motile actin - long known as a hallmark of cancer cells and the property that makes them look transformed to the human eye - is, in fact, playing a major role in regulating cancer metabolism," says Lewis C. Cantley, Ph.D., Meyer director of the Sandra and Edward Meyer Cancer Center, who led the study with Gerburg Wulf, M.D., associate professor of medicine at Harvard Medical School.
The study, published Jan. 28 in Cell, further elucidates the biology underlying the role of phosphoinositide 3-kinase (PI3K) in glycolysis, the metabolic process that enables cancer cells to thrive by generating biomass and energy.
PI3K is a cell-signaling molecule discovered by Dr. Cantley 28 years ago that has been implicated in a large number of cancers. It was known to have a vital role in sugar breakdown, but this study shows that it also plays a part in cellular architecture.
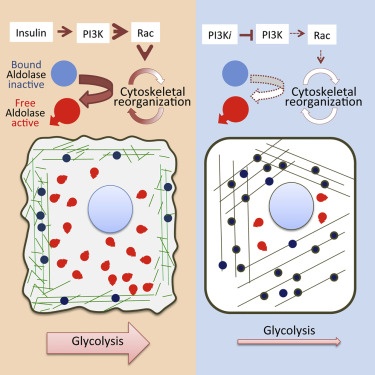
In a normal cell, signaling from outside activates the cytoskeleton, the highly organized and flexible framework of the cell. The cytoskeleton is not static, it's a dynamic assembly of fiber bundles that undergo constant turnover, breaking down the energy molecule ATP in order to maintain the cell's shape.
These fibers are composed of the protein actin. The researchers discovered that when PI3K is activated and ATP breakdown is accelerated, as occurs in cancer cells, the actin fibers fall apart and release an enzyme called aldolase.
"It turns out that enzymes sit on these actin fibers, like birds might sit on a telephone wire," said Wulf. "If the wire is cut, the birds fly away. Similarly, when actin fibers are broken down, the aldolase enzymes fall off. We discovered that it's the process of 'falling off' that activates aldolase - and accelerates glycolysis."
When PI3K functions normally, it regulates key cell functions including growth, motility, proliferation and differentiation. But an overactive PI3K signaling pathway can contribute to the development of cancer. Inhibiting PI3K in turn inhibits glucose breakdown, starving cancer cells of the sugar they need for self-renewal.
Based on these observations, the development of PI3K inhibitors has been a major focus of personalized cancer therapies. Wulf said this new discovery will provide essential new information for drug development, and could help identify companion therapies that may be more effective than PI3K inhibitors alone.
“As we continue our investigations of PI3K, it's becoming clear that blocking this pathway alone is not enough to stop cancer growth and that PI3K inhibitors may be drugs that need to be combined with other drugs to effectively and specifically block tumor cell metabolism,” she said.
Other Weill Cornell authors include Mark Lundquist, Ph.D., and Costas A. Lyssiotis, Ph.D. (now at University of Michigan). The study was funded in part by: the National Institutes of Health, The National Science Foundation; the United States – Israel Binational Science Foundation; Stand Up to Cancer; the Breast Cancer Research Foundation; the Mary Kay Ash Foundation; the Men’s Initiative of DFHCC; the Breast Cancer Alliance; and the Damon Runyon Cancer Research Foundation.