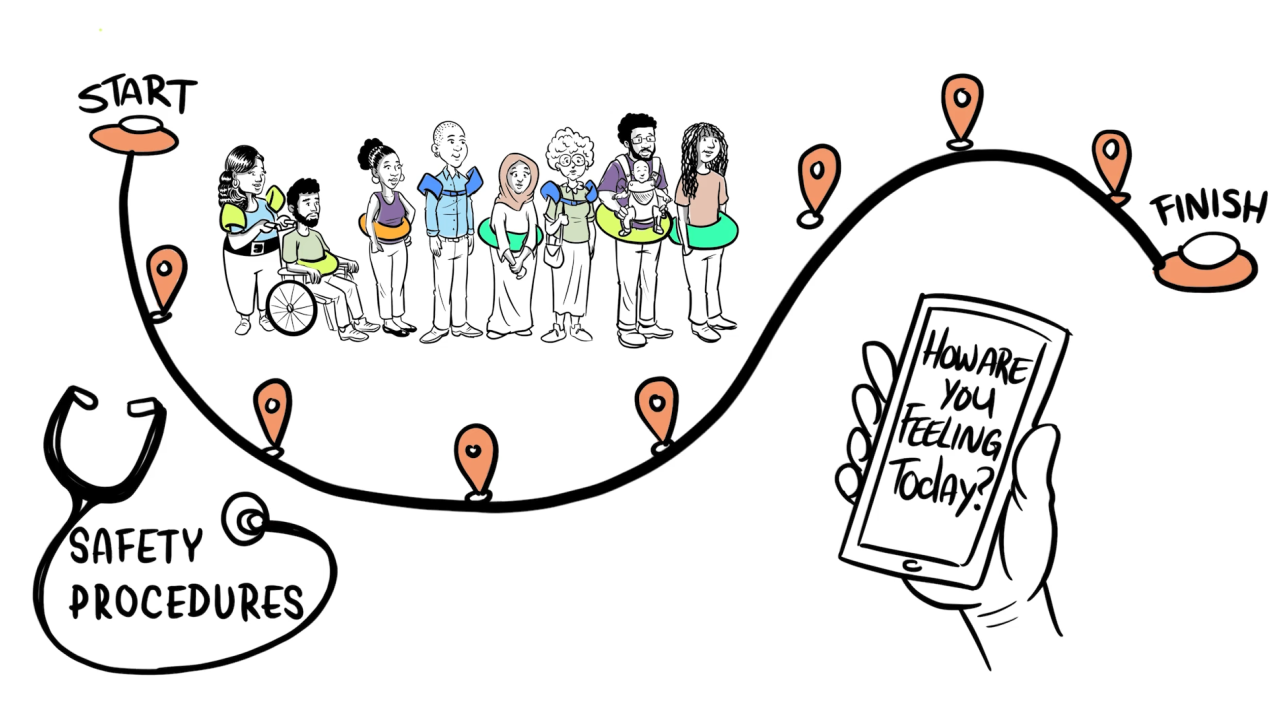
Safety is Paramount
The thought of joining a clinical trial may feel scary. You may be wondering:
- Are there risks to taking part in the trial?
- Who is watching out for any problems?
- Who is making sure that people who take part are safe?
- Is the trial trying to answer an important research question?
In fact, the Meyer Cancer Center adheres to the most rigorous review and monitoring process, including complying with all federal rules to ensure the safety and ethics of clinical trials. To keep you safe, trials are reviewed before they open by scientific experts and an institutional review board (IRB). Once the trial starts, it is continuously monitored by the institutional review board (IRB), Data and Safety Monitoring Committee (DSMC) for phase 3 trials, organization sponsoring the trial, and the research team.
Mechanisms to Protect Patients
Text adapted from the National Cancer Institute; images provided by Genentech. Reviewed on November 15, 2023.