From tracer to treatment: Radiopharmaceuticals home in on hard-to-detect cancers
In order to understand and treat a tumor, you must first find it.
John Babich, Ph.D., professor of Radiopharmaceutical Sciences in Radiology, has spent much of his career devoted to developing novel molecular imaging tools for medical diagnostics and therapeutics.
Prior to joining Weill Cornell Medicine in 2013, Babich spent 15 years in the pharmaceutical industry. In 1997, he co-founded the company Molecular Insight (acquired by Progenics in 2013), and created a series of radiolabeled small molecules that could be used to visualize and treat prostate cancer.
Babich said he was inspired by the work of his current Weill Cornell Medicine colleagues, Neil Bander, M.D., and Scott Tagawa, M.D., who pioneered the use of radiolabeled monoclonal antibodies for imaging and therapy of prostate cancer. Dr. Bander’s antibody, J591, binds to prostate-specific membrane antigen (PSMA), a transmembrane protein that shows a significant over-expression on prostatic cancer cells and especially in advanced stage prostate carcinomas, with low expression in normal human tissue. Uniquely, PSMA expression levels continue to rise if the prostate cancer spreads to other organs, so it is also a good diagnostic for visualizing metastatic spread. In collaboration with their colleagues in nuclear medicine, Stanley Goldsmith, M.D., and Shankar Vallabhajosula, Ph.D., they have demonstrated that PSMA is an excellent target in this disease.
“There is high expression of the protein in prostate cancer. We are exploiting this expression to the cancer cells’ own detriment,” Babich said. “I wondered what the possibilities might be to exploit PSMA even further using small molecules.”
His approach focuses on enzyme inhibitors rather than antibodies, using radioactive ligands to trace these enzyme inhibitors as they bind to the protein, similar to how radioactive iodine can be used to illuminate thyroid cancer. Using the right combination of radionuclides, such as gallium 68, the distribution of the ligand is visible inside the body via positron emission tomography (PET scan).
Working closely with his urology, oncology and surgical colleagues across the Meyer Cancer Center – including Tagawa, Douglas Scherr, M.D., Jim Hu, M.D., and David Nanus, M.D. – as well as other members of the Molecular Imaging Innovations Institute, Babich has developed a protocol that combines his radiopharmaceuticals and PET scans to improve detection and therefore patient management in prostate cancer.
“If you can see tumors when they are at a low-grade disease stage, there is a high chance for a cure, but as you go up in grade those odds drop. If the cancer has metastasized, the man has only about a 30 percent five-year survival rate,” Babich said.
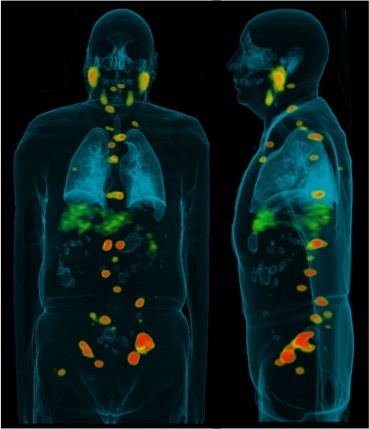
Not only can Babich’s protocol detect cancer earlier, it also catches smaller amounts of cancer that might have been missed by conventional imaging techniques, or that have spread elsewhere.
“We are the first in the city to have this technology available,” he added. “It enables us to see the disease much better than what is currently possible using an MRI scan, and we have the potential to do imaging throughout the treatment process, which helps guide and expand future treatment plans.”
A clinical trial at Weill Cornell Medicine has already resulted in several success stories. One involved a 63-year-old man who had already undergone radiation and hormone therapy. His PSA (prostate-specific antigen) levels were high, and a biopsy of his prostate showed evidence of cancer, so surgery was scheduled to remove the gland. CT scans showed no indications that the cancer had spread elsewhere, but radioimaging based on the Babich protocol revealed metastasis in several lymph nodes and the abdomen.
“That changed the patient’s care radically,” Babich said.
From tracer to treatment
Another exciting element of Babich’s radionuclide approach is the ability to be converted from imaging tools to treatment tools, delivering highly targeted therapeutic doses of radiation to kill the tumor.
He does this by changing the radionuclide attached to the ligand. He starts with a base molecule, then chemically creates a chelate “basket” to grab metals (such as nuclear isotopes), which it then stably holds via ionic bonding.
Different isotopes emit radioactive signals of different qualities and intensities. Gallium-68, for instance, is a positron emitter with a relatively short half-life (the amount of time it takes for it to lose its radioactivity) of 68 minutes, so it is ideal for PET imaging. By swapping gallium for Lutetium-177, a beta emitter, into the chelate basket instead, Babich can deliver therapeutic beta radiation and extend the radioactive emissions for six days or more.
His previous commercial ligands and protocol are about to enter Phase III clinical trial testing, and Babich is now focused on developing the next generation of radiopharmaceuticals for prostate cancer. He is very keen on introducing alpha particle therapy to the small molecular constructs. By emitting alpha particles instead of beta particles, the compounds would deliver a much stronger dose to the tumor and allow for more precise targeting.
“It’s like the difference between a cannon ball and a bullet,” Babich said. “You can break a lot of things with a cannon ball. We want to minimize collateral damage caused by beta particles.”
But alpha particles are also much more toxic - there is a high risk of damaging the salivary glands, where the PSMA ligands also tend to accumulate once administered, for instance - so Babich’s team is experimenting with ways to overcome such challenges.
PSMA appears on the vessels of brain tumors as well. In a recent collaboration with neuro-oncologist Howard Fine, M.D., director of the Weill Cornell Brain Tumor Center and associate director for translational research in the Meyer Cancer Center, Babich is exploring the use of his tracer to image the vascular beds of gliomas along with his radiology colleague Gloria Chiang, M.D.
There's an(other) app for that
The radiochemist is also investigating the use of PET tracers in other tumors, such as neuroendocrine cancers.
“These tumors frequently display receptors for the peptide somatostatin.” Babich said. “We have initiated a clinical trial of a gallium-68 labeled sandostatin analog which shows excellent imaging characteristics for the detection of a broad range of neuroendocrine tumors such as carcinoid and pancreatic neuroendocrine cancer.”
Neuroendocrine tumors involve hormone-producing endocrine cells and nerve cells which can manifest itself in a variety of organs, with varied and often debilitating symptoms. Often, they are tiny, amorphous lesions that move around inside the gut, making them extremely difficult to find.
“You can cure them if you can find the disease and cut it out. But up until now, that’s been a real challenge,” Babich said.
He may also expand his compound library to study the metabolism of gliomas. It is possible to develop radiolabeled analogues of sugars and fats and amino acids that can be used for detailed study of tumor metabolism.
This information would not only advance our understanding of a cancer that is difficult to study, it could also inform treatment. It could help characterize the aggressiveness of the tumor, and test the hypothesis that starving tumors of glucose might kill them.
“My goal is to bring new ways of seeing disease in patients to my research and clinical colleagues,” Babich said. “It can help researchers understand the basic science, and give really unique tools to clinicians.”