Skinny Fat
As the nation’s waistlines expand, so too is the evidence linking obesity with increased risk of several cancers, and worse prognosis. But even those who maintain a “healthy” weight may be harboring hidden metabolic problems that make them more likely to develop cancer.
Early results of studies led by Andrew Dannenberg, M.D., found that approximately one-third of normal-sized women had inflammation in their breast tissue, a condition connected to cancer and a potential sentinel of chronic conditions elsewhere in the body.
“People don’t know they have this. If you hurt your knee, you know it. If you have adipose inflammation, you don’t know it. The public health significance of unrecognized chronic inflammation is likely to be profound.”
Dannenberg, the Henry R. Erle, MD-Roberts Family Professor of Medicine at Weill Cornell Medical College and Associate Director of Cancer Prevention at the Sandra and Edward Meyer Cancer Center, has spent years investigating inflammation, which happens when the body’s immune response is activated.
By 2009, the scientific community had established links between chronic inflammation of fat tissue with cardiovascular disease and diabetes.
“The connection to cancer seemed potentially important, but it was not in the literature,” Dannenberg said. Encouraged by funding from the Breast Cancer Research Foundation to ask innovative questions, he initiated this new line of investigation.
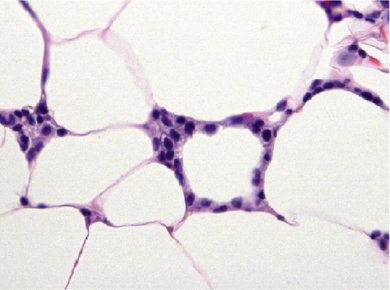
The fat cells in these tissues – white adipose tissue – grow much larger than normal fat cells. Inadequate oxygen supply appears to contribute to the death of those enlarged cells. As they die off, the body’s immune system responds, sending in special white blood cells (macrophages) to gobble up the adipocytes and clear them away. The macrophages form an envelope around the dead or dying adipocytes, creating a crown-like structure (CLS), a hallmark of inflammation.
Working closely with Kotha Subbaramaiah, Ph.D., the Jack Fishman Professor of Cancer Prevention at Weill Cornell Medical College, Cliff Hudis, M.D., Chief of Breast Medicine Service, and others at Memorial Sloan Kettering Cancer Center – a relationship that has continued to this day - he set out to elucidate such a link, starting with post-menopausal breast cancer, where there was a well-described connection between obesity and estrogen-dependent forms of the disease. Dannenberg had previously worked on the gene that makes estrogen (aromatase), and he found it in elevated levels in the breast tissues of obese women.
It was thought that elevated body mass index (BMI) would be a good indicator of the presence of white adipose inflammation. But a recent study from the Weill Cornell/Memorial Sloan Kettering team had a surprising finding: white adipose tissue inflammation occurs in women with normal BMI, too.
Although the initial sample size was relatively small – 100 women – the results were striking. Ninety percent of obese women had evidence of white adipose tissue inflammation as defined by the presence of crown-like structures. But so did about a third of normal-sized women: 17 of 48 of normal-sized women were inflamed, with large fat cells that resembled those of obese women.
The normal-sized women with inflammation were more likely to be insulin resistant than their counterparts without inflammation. They also had numerous changes in circulating metabolites that correlated with occult (asymptomatic) breast inflammation.
High levels of insulin in the blood have been known to be associated with bad outcomes in breast cancer, and a recent study by Imperial College London’s Marc J. Gunter, Ph.D., et al found that elevated insulin levels could be a risk factor even in normal-sized women. Another study by National Cancer Institute investigator Britton Trabert, Ph.D., found that a diagnosis of metabolic syndrome was associated with higher risk of endometrial cancer, regardless of whether the woman was considered obese. Dannenberg suspects that occult adipose inflammation is contributing to insulin resistance and the development of cancer even in normal-sized women.
“The good news is, this is potentially targetable, with diet, lifestyle and pharmacological interventions.”
Caloric restriction has been shown to shrink adipose cells and decrease inflammation in mice, he said. Maintaining a healthy lifestyle is one of the best strategies for cancer prevention, and Dannenberg said these revelations about the hidden risk among those who appear healthy underscores its importance even more.
Dannenberg and his collaborators at Memorial Sloan Kettering have now expanded their breast cancer inflammation study to include another 100 women.
Expanding focus
A $1 million grant from the Prostate Cancer Foundation is also enabling the research team to apply their discoveries to prostate cancer, where the existence of excess fat around the organ – periprostatic adipose – has been connected to more aggressive disease.
They have already found a correlation between inflammation and high-grade prostate cancer. Consistent with the findings in the breast adipose study, approximately one-third of normal-sized men had evidence of periprostatic inflammation.
“More evidence that you can’t judge by size,” Dannenberg said.
Obesity has also been linked to worse prognosis in squamous cell carcinoma of the tongue, an organ with its own adipose tissue. Dannenberg is studying whether inflammation is the key culprit, and early indicators suggest it does play a role.
In a study recently presented at the European Society of Medical Oncology (ESMO), crown-like structures of the tongue were linked with poorer outcomes. This suggests that in the case of individuals with tongue inflammation, clinicians may want to reconsider the “watchful waiting” approach often taken when a patient appears to be free of disseminated disease at the time of surgery.
In such cases, determining whether inflammation is present could be invaluable to help direct clinical decisions, Dannenberg said.
Since occult adipose inflammation is common and associated with diabetes, cardiovascular disease and cancer, non-invasive diagnostic tests are needed. Working with Xi Kathy Zhou, Ph.D., associate professor of biostatistics in healthcare policy and research, and the Memorial Sloan Kettering team, he has identified a handful of molecules in blood that appear to be sensitive and specific when it comes to identifying white adipose tissue inflammation. Armed with this information and a $250,000 grant from the Prevent Cancer Foundation, his team is carrying out a validation study. The long-term goal is to develop a blood test to diagnose inflamed fat, which would be more reliable than BMI, much less invasive than a breast biopsy, and could have future applications for other conditions.
The biology of inflamed adipose tissues is complex, with numerous differentially expressed genes, Dannenberg said. Identifying the mechanisms by which it may initiate or drive disease has been difficult, but he believes it may be common across many cancers. His hope is that an increased understanding of local and systemic consequences of adipose inflammation will lead to new risk reduction and treatment strategies for patients.
“I think we’re proving that adipose inflammation is contributing to tumor progression, by helping to create a tumor-promoting microenvironment,” Dannenberg said. “Adipose inflammation could prove to be clinically important for the development and progression of a range of cancers. There’s strong clinical evidence for tongue and breast, and what could be more different than tongue and breast?”