Structural Study Points the Way to Better Malaria Drugs
Structural insights into a potent antimalarial drug candidate’s interaction with the malaria parasite Plasmodium falciparum have paved the way for drug-resistant malaria therapies, according to a new study by researchers at Weill Cornell Medicine and Van Andel Institute.
The antimalarial molecule, TDI-8304, is one of a new class of experimental therapeutics that targets the proteasome, an essential, multiprotein complex in P. falciparum cells. Two years ago, the researchers showed in a preclinical study that TDI-8304 potently kills malaria parasites at multiple stages of their life cycle and clears parasites in animal model of malarial infection.
In the new study, published Dec. 14 in Nature Communications, the researchers used cryo-electron microscopy (cryo-EM) to show how TDI-8304 fastens to its proteasome target. The high-resolution imaging also revealed what happens to P. falciparum, the major cause of malaria deaths, when it mutates to become less sensitive to TDI-8304.
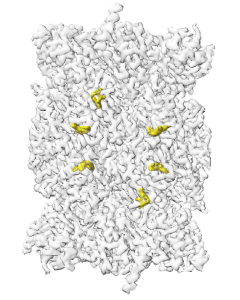
This high-resolution map depicts the antimalarial drug candidate TDI-8304 (in yellow) as it fastens to its target in the malaria parasite, Plasmodium falciparum. Understanding this critical interaction may aid in the development of new anti-malaria medications that are less susceptible to resistance. Image courtesy of the Li Lab, Van Andel Institute.
“This study gives us insights that should help in the development of new proteasome-inhibitor antimalarials that are not very susceptible to resistance,” said study co-senior author Dr. Gang Lin, an associate professor of research in microbiology and immunology at Weill Cornell Medicine.
“Remarkably, the mutation that makes P. falciparum less sensitive to TDI-8304 makes it more sensitive to compounds that target a different part of the parasite proteasome,” said co-senior author Dr. Huilin Li, professor and chair of the Department of Structural Biology at Van Andel Institute. “Our cryo-EM analysis illuminated how that ‘collateral sensitivity’ occurs.”
The study’s first author was Dr. Hao-Chi Hsu, a research scientist in Dr. Li’s lab at Van Andel Institute. Dr. Laura Kirkman, an associate professor of medicine and microbiology and immunology at Weill Cornell Medicine, also collaborated on the study.
A Need for New Anti-Malaria Strategies
Malaria continues to be a major public health threat, with roughly 250 million cases per year, and more than 600,000 deaths, principally in young children. Although malaria vaccines have been developed—the World Health Organization currently recommends two of them—their effectiveness and uptake in malaria-endemic regions are far from ideal. The widespread use of antimalarial drugs also has led to the emergence of drug resistance in malaria parasites. Thus, new antimalarial drugs that are durably effective and available in pill form would be particularly useful.
In 2014, Dr. Lin and Dr. Kirkman began collaborating to develop molecules that inhibit the activity of the P. falciparum proteasome without affecting the human counterpart. They began working with Weill Cornell Medicine’s Enterprise Innovation team, and ultimately began a collaboration with the Sanders Tri-Institutional Therapeutics Discovery Institute (Tri-I TDI), which partners with faculty at Weill Cornell Medicine, Memorial Sloan Kettering Cancer Center, and The Rockefeller University to translate groundbreaking biological discoveries into small molecule or biologic therapeutics.
TDI-8304 emerged from that effort. In a 2021 study, Dr. Lin and colleagues showed in lab-dish and preclinical model tests that TDI-8304 is highly effective, even against P. falciparum strains that are resistant to first-line antimalarials called artemisinins. However, their early work showed that P. falciparum can acquire mutations that reduces the parasite’s susceptibility to molecules like TDI-8304, which primarily targets one subunit of the parasite proteasome.
Structural Insights Reveal Strengths and Weaknesses of Drug Candidate
The main aim of the new study was to determine how TDI-8304 binds to the subunit, how that target differs on the human proteasome, and how the parasite’s new mutation enables resistance. For this, Dr. Lin turned to his long-time collaborator Dr. Li, a structural biology expert.
Cryo-EM studies of malaria parasite structures tend to be challenging, due to the difficulty of growing the parasites in blood cells and enriching sufficient material. But Dr. Li and his team succeeded in solving the TDI-8304/proteasome interactions at very high resolution.
These models showed that TDI-8304 makes extensive connections to its target, whereas the new mutation, which “flips” a portion of the adjacent subunit, greatly weakens those connections.
Dr. Kirkman’s prior work had shown that this mutation also somehow enhances the potency of a compound targeting a different part of the proteasome. In the new study, the resolution of the structure of this compound bound to the mutant proteasome clarified how this happens.
The researchers hope to use these findings to develop therapeutics that selectively and potently hit both these proteasome targets—a strategy that should delay the emergence of resistance. Drs. Lin and Li envision that such an approach could be combined with existing drugs to further minimize the risk of resistance.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, see the profile for Dr. Gang Lin.
Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award nos. R01AI143714 (Lin) and R01AI070285 (Li). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.



