First clinical trial of new targeted molecular therapy in U.S. takes aim at incurable prostate cancer
NewYork-Presbyterian and Weill Cornell Medicine have begun the first clinical trial in the United States that uses a radiopharmaceutical to treat men with progressive prostate cancer that has spread beyond the prostate and is no longer responding to hormonal therapy. The Phase 1 study has completed its second round of patient enrollment, with the first six patients having undergone dosing. The researchers will be discussing the trial on June 5 at the 2017 American Society of Clinical Oncology meeting in Chicago.
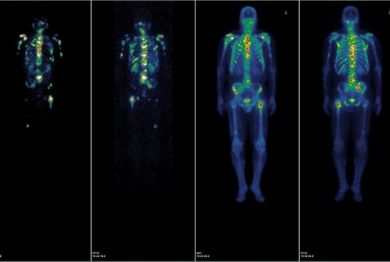
The researchers are using the small molecule Lutetium 177Lu-PSMA-617 to target prostate-specific membrane antigen (PSMA), a protein that is abundantly expressed in 85-90 percent of metastasized prostate cancers. The small molecule binds to PSMA and delivers precise radiation therapy intended to shrink the cancer — even in cases in which cells have yet to form a visible tumor on a bone or CT scan.
The trial primarily seeks to determine the highest dose level of the drug that can be given without significant side effects. PSMA-targeted therapy is thought to be one of the most promising approaches in treating metastasized prostate cancer.
“This trial represents a new frontier in the treatment of metastatic prostate cancer,” said Dr. Scott Tagawa, medical director of the genitourinary oncology program at NewYork-Presbyterian/Weill Cornell Medical Center and The Richard A. Stratton Associate Professor in Hematology and Oncology at Weill Cornell Medicine. “While this type of therapy has shown promise, this is the first trial of its kind in the United States. So far, patients are doing well.”
While this trial is the first of its kind in the United States, this same approach to treat metastatic prostate cancer has gained traction in recent years in Germany, where physicians can treat patients who have exhausted standard treatment options under “Compassionate Use” laws. German physicians who are able to provide this treatment in a “Compassionate Use” setting have shown Lutetium 177Lu-PSMA-617 can reduce the volume of tumors in the body and lead to remission of the cancer.
NewYork-Presbyterian and Weill Cornell Medicine have been at the forefront of PSMA-targeted 177Lu therapy for more than a decade. Dr. Neil Bander, the Bernard and Josephine Chaus Professor of Urological Oncology at Weill Cornell Medicine and a urologic oncologist at NewYork-Presbyterian/Weill Cornell Medical Center, developed the first monoclonal antibodies that could bind to PSMA in prostate cancer cells. As a result of Dr. Bander’s efforts, PSMA has become recognized as the best known prostate-cancer specific cell surface molecular target. The lead antibody he developed, J591, was shown to be able to target virtually all prostate cancers in patients while also avoiding healthy tissue and normal organs.